How to Scale-Out and Scale-Up Cell Therapy Production
By Dr. Hue-Tran Hornig-Do.
The field of cell therapy is advancing rapidly and meeting growing demand poses major challenges for manufacturers. Efficient manufacturing is a crucial aspect of deciding whether to commercialize a cell therapy; however, expanding the number of living cell-based medical products to a large patient population is not without challenges. Since the cells are the therapeutic product, they need to retain their phenotype and functionality at each stage of the process, regardless of the manufacturing method.
Scale Up or Scale Out
There are two widely used strategies for generating large numbers of cells: scale-up and scale-out. Scale-up systems are based on the use of larger vessels to increase production capacity, while scale-out systems increase capacity using multiple culture vessels working in parallel.1,2 Both strategies have their advantages and disadvantages and are not necessarily mutually exclusive. Indeed, comprehensive workflows often include both. Scale-out can be straightforward because the production unit remains the same. However, reproducibility can be difficult to achieve. Scale-up, on the other hand, can be more complex and requires more planning, but it can lower the costs of the product in the long term. Which strategy you should use depends on how much material is needed for the therapeutic being manufactured. Let's take a closer look at this question using autologous and allogeneic cell therapy as examples.
Autologous cell therapies are examples of personalized medicine that use a patient’s own cells as the starting point and are manufactured as a single lot per patient.3 Here, the concept of scale-out can be applied where several parallel production lines are set up for individual patient products and run simultaneously in the same production facility. Such a scale-out manufacturing approach can also be transferred to other manufacturing facilities to increase output. For example, this model is currently being adopted by chimeric antigen receptor (CAR) T-cell biotech companies, through the setting up of regional manufacturing facilities in the United States and Europe, as well as expansion to other regions. On the other hand, allogeneic therapies are manufactured in large quantities from unrelated donor tissues and are considered an “off the shelf” product used to treat many patients.4 These therapies require a scale-up approach by increasing the manufacturing vessel volume.
Adherent or Suspension Platform
Which scale-up method is the best for my approach? Whether you should choose a suspension or an adherent platform basically depends on the cell type, as cell culture platforms largely try to mimic the native environment for the type of cell being grown. For example, cells from solid tissues such as muscle are more suitable for adherent culture, while cells from liquids such as blood are more suitable for suspension culture. CAR T-cells are nonadherent and can be cultured in suspension systems, while stem cells that mostly exist in contact-dependent niches (where they are in direct contact with other cells and extracellular matrices) can be successfully expanded using adherent platforms.
A key advantage of adherent platforms is the ability to utilize various surface modifications that mimic a local micro-environment. In some cases, adherent systems also offer direct visualization, for example, cell growth in flasks or stacked vessels can be easily monitored under the microscope. Adherent cultures also give the gift of time, a significant benefit for start-ups that want to get to market quickly. These and other advantages make adherent cell culture systems a good choice for many cell and gene therapy programs, which tend to involve anchorage-dependent cell types.
However, scale-up in a suspension platform has long been considered the preferred method. One reason for this is that suspension cultures can be grown in conventional, large-scale stirred-tank bioreactors, which reduces the number of processing steps and decreases the footprint of the facility. That said, the scaling-up of suspension-cultured cells to larger vessels also presents some pitfalls. Careful agitation is required to ensure adequate gas and nutrient exchange, while minimizing cell damage induced by shear stress.5 A thorough evaluation of culture flow dynamics based on equipment specifications (e.g., the number of impellers, the stirring speeds, and the mixing strategies) is essential to determine potential effects on cells. Despite these challenges, suspension cultures offer scalability and can produce the high volume of cells needed for applications. This scalability and control make suspension platforms an attractive option for manufacturers looking to increase operational efficiency.
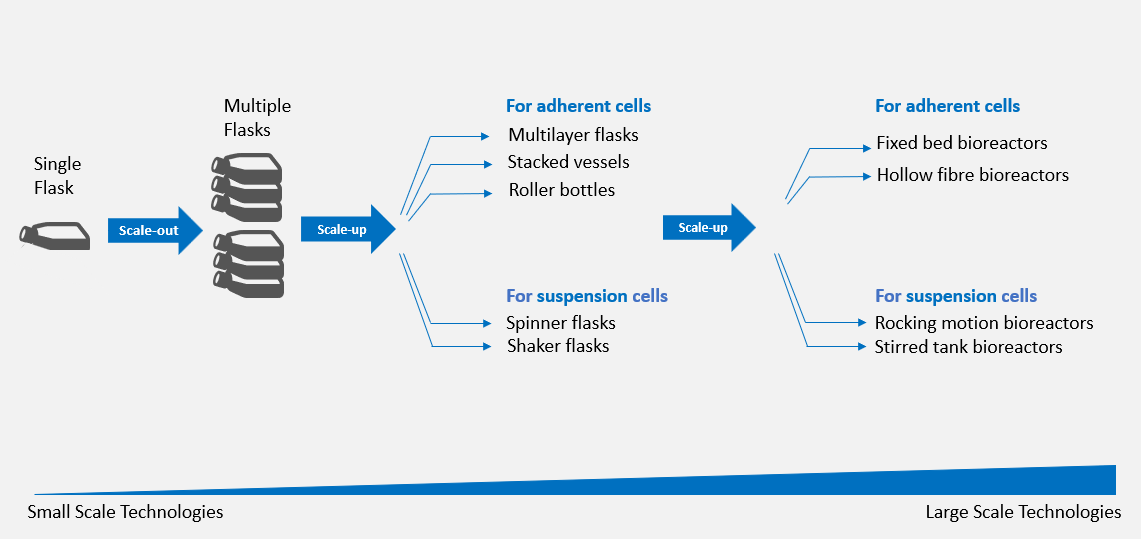
Figure 1: Systems for Scaling Up Both Adherent and Suspension Cell Cultures
Is it possible to coax adherent cells to shift modalities and make them amenable to suspension culture conditions? The answer is yes, and a classic example is the production of monoclonal antibodies using Chinese hamster ovary (CHO) cells. Researchers have modified these cultures to thrive in a suspension environment - and today they are mainly produced in this way. However, this often requires extensive adaptation and sometimes genetic modification of adherent cell lines to make them suitable for suspension culture conditions. This takes time and more work, and the question is whether the enormous effort is worth it for your needs. Not every approach needs that kind of scale. Autologous therapies and gene therapies targeting rare diseases or small patient population sizes may be produced in sufficient quantities at a smaller scale. In addition, great strides have been made in the development of adherent cell culture platforms, leading to newer technologies such as microcarriers and fixed bed bioreactors that make it possible to achieve similar economies of scale with adherent culture as with suspension methods.
Culture Systems From Small to Industrial Scale
Cells cultivated in small-scale culture systems, such as a single T-flask, can be scaled-out in multiple flasks. At a bench scale, the transition to larger vessels can be used to scale-up a process. Multi-layered flasks, stacked vessels, and roller bottles are the most used devices to scale-up adherent cultures, while spinner flasks and shaker flasks are a good choice for scaling-up suspension cells.
When entering into industrial-scale processes, rocking motion bioreactors, stirred tank bioreactors, packed bed (also called fixed bed) bioreactors, and hollow fibre bioreactors are the four most used devices to scale-up cells. Rocking motion and stirred tank bioreactors are widely used for the expansion of suspension cells such as T-cells,6 while structured-support bioreactors such as fixed bed and hollow fibre bioreactors have been employed to expand adherent cells like mesenchymal stem cells (MSCs) and human umbilical cord derived hematopoietic stem cells (HSCs).7,8
As noted above, microcarriers are small spheres that are available in various sizes and materials, with various coatings, which allow for the growth of adherent cells in a suspension system. They can be added to spinner flasks to generate a hybrid adherent/suspension system and are used mainly to perform preliminary tests before moving to larger stirred tank bioreactors.9
In summary, there is no “one-size-fits-all” platform and different technologies certainly offer different benefits, with different weightings depending on your resources and scale, as well as your visualization and automation needs. Ultimately, it's about finding out what your needs are and what you expect from your platform.
We at the Fisher Scientific channel are here to help you figure out which solution can best support your goals. Our aim is to help researchers and manufacturers advance innovative cell therapies by providing trusted products and tools, including a wide range of innovative culture systems from leading manufacturers.
References
1. Roh K-H, Nerem RM, Roy K. Biomanufacturing of therapeutic cells: state of the art, current challenges, and future perspectives. Ann Rev Chem Biomol Eng (2016) 7: 455–478.
2. Macdonald GJ. Scale-out plus single-use can multiply yields: single-use technology reduces some biomanufacturing equations to scale-out > scale-up. Genetic Eng Biotechnol News (2019) 39: 46–48.
3. Fesnak AD. The challenge of variability in chimeric antigen receptor T cell manufacturing. Regen Eng Transl Me. (2020) 6: 322–329.
4. Caldwell KJ, Gottschalk S, Talleur AC. Allogeneic CAR cell therapy-more than a pipe dream. Front Immunol (2021) 11: 618427.
5. Eaker S, Abraham E, Allickson J et al. Bioreactors for cell therapies: current status and future advances. Cytotherapy (2017) 19: 9–18.
6. Smith TA. CAR-T cell expansion in a Xuri cell expansion system W25. Methods Mol Biol (2020) 2086: 151–163.
7. Park S, Stephanopoulos G. Packed bed bioreactor with porous ceramic beads for animal cell culture. Biotechnol Bioeng (1993) 41: 25–34.
8. Gagliardi C, Khalil M, Foster AE. Streamlined production of genetically modified T cells with activation, transduction and expansion in closed-system G-Rex bioreactors. Cytotherapy (2019) 21: 1246–1257.
9. Bodiou V, Moutsatsou P, Post MJ. Microcarriers for upscaling cultured meat production. Front Nutr (2020) 7: 10.




22_04.jpg-650.jpg)