Growing Replacement Organs in a Culture Dish
By Dr. Hue-Tran Hornig-Do.
What Are Mini Organs and How Are They Created?
Does producing miniature versions of organs in a dish sound like science-fiction? Thanks to advances in stem cell technology and bioengineering scientists are now able to artificially grow organoids with similar properties to organs. Here is my experience of seeing a mini organ for the first time in my life: I was amazed and shocked at the same time when I looked at the lump of cells under the microscope that beat like the real thing. They are pulsing, and what a hypnotic rhythm. Incredible! “What is this?” I excitedly asked my colleague who was presenting me with her study’s latest success. “These are human mini hearts I have been creating for three months.” Said my colleague, a stem cell biologist whose dream it is to cure heart failure by using stem cells.
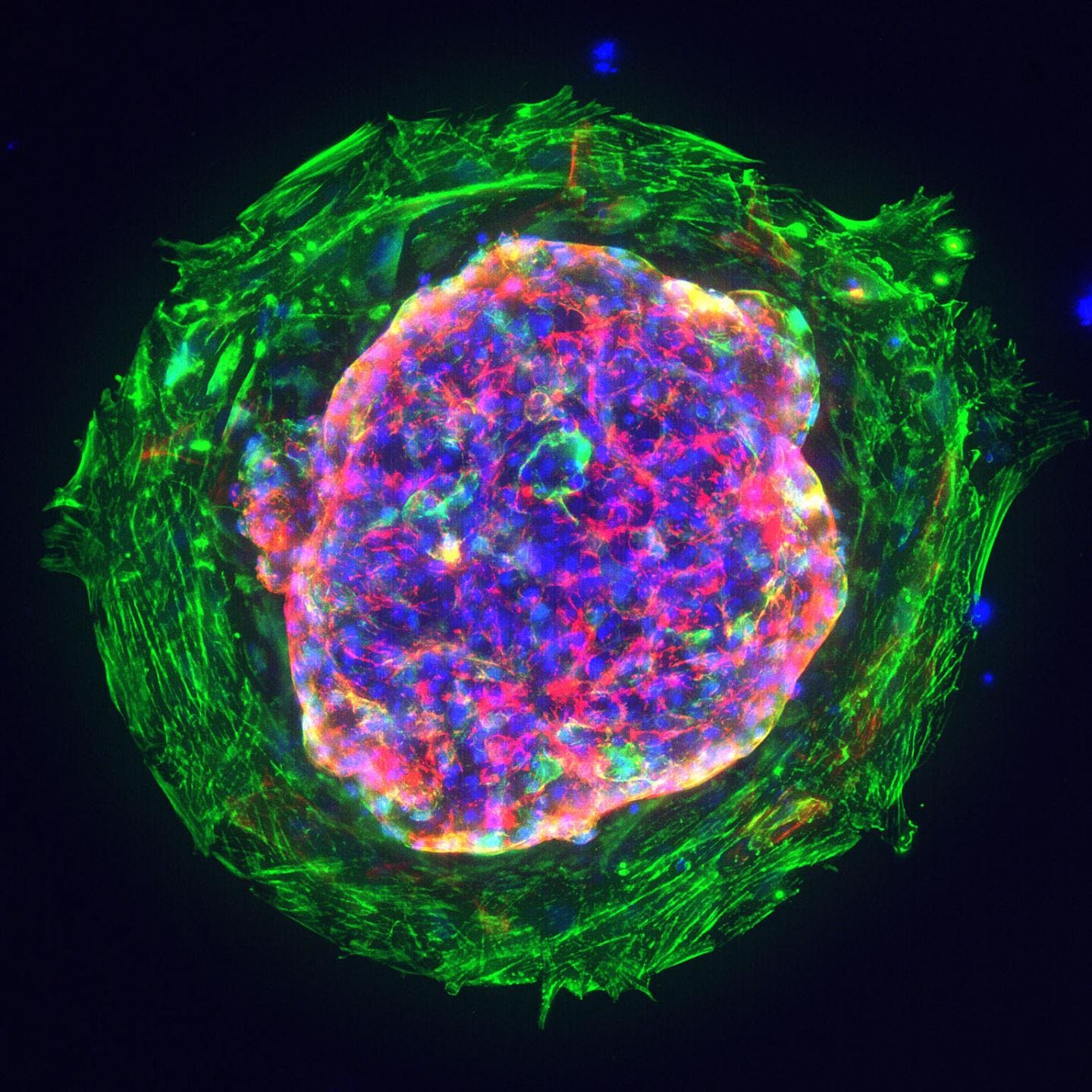
Figure 1: Heart microchamber generated from human iPS cells; cardiomyocytes (red), myofibroblasts (green), cell nuclei (blue); Source: Zhen Ma, University of California, Berkeley.
With a diameter of about 1 or 2 millimetres, the mini hearts, also known as cardiac organoids, seen under the microscope, were no larger than sesame seeds. They contained the main types of cells typically present in this stage of development and had a clearly defined chamber that beat at 60 to 100 times per minute, the same rate of an embryo's heart around the same age. Organoids are a three-dimensional assembly of cells, mimicking the structure, function, gene specificity and other characteristics of the source tissue.1 They are derived from stem cells that have the ability to develop into different types of cells in the body.
To obtain stem cells, my colleague isolated skin cells from different donors and reprogrammed them into versatile stem cells called iPSCs (induced pluripotent stem cells).2 The iPSCs were then differentiated into cardiomyocytes and other cells from which a human heart can mature by adding various essential growth factors, signal substances and matrices.3 “It was like a miracle: the stem cells have organised themselves into a three-dimensional structure that resembled the heart. Just like that. However, my mini hearts cannot pump blood, not yet.” Commented my colleague with a smile. Since then, stem cell research has been rapidly evolving, and researchers have been able to produce organoids that closely resemble the brain, kidney, lung, intestine, retina, stomach, and many more.1
What Do We Learn From Growing Organoids?
By creating organoids from a patient's own stem cells, scientists can study how organs form and grow, providing them with new insights on human development and disease mechanisms. Organoids can be used to study the complex arrangements and interactions of cells in three-dimensional space, which is not possible with most other experimental models.
The mini organs have shown promise as new models for drug discovery and development, where numerous compounds need to be tested on cells to see how they work. Until now, the pharmaceutical industry has relied on animal models and human cell lines for such tests, which bear little resemblance to normal or diseased tissue. By using organoids, we can learn how an individual's organs respond to different drugs or treatments. This could lead to more targeted and effective therapies, tailored to each patient's unique needs. Finally, the usage of organoids helps to alleviate ethical problems - for example, by replacing animal testing.
Can We Use Organoids to Grow Tissue For Transplantation?
For some time, regenerative medicine has faced issues such as donor shortage, immune rejection and ethical questions that are driving the search for alternatives.
"Using stem cells to repair or replace damaged or diseased tissues and organs is a promising alternative."
Organoids can be rapidly produced in vitro and retain the 3D structure, function, heredity, and phenotypic specificity of the original tissue. These advantages are critical for transplantable, immune, and functional tissues or organs, which undoubtedly heralds a new dawn for regenerative medicine. Indeed, organoids of murine intestines4, livers5, kidneys6 and pancreas7,8 have been successfully transplanted into mice with restoration of organ function in experimental conditions. Furthermore, the use of IPSC-derived corneal cell transplantation has already began in a trial in Japan.9 However, there is still a long way to go before organoids may serve as autologous replacement organs in the future.
What Are The Challenges That Lie Ahead Before Clinical Application?
Organoids are still too small, and there is still a discrepancy between their functions and those of normal tissue. When organoids grow to a certain extent, the cells in the centre can no longer be adequately supplied with nutrients, and the elimination of metabolic waste in the cells is difficult because most organoids lack vasculature cells. Indeed, the vascular organisation of future organoids poses a major challenge. Similarly, it is necessary to provide an adequate innervation system in the organoids of the future. Scientists are currently developing various methods to integrate other cell systems, such as the vasculature, into organoids. In summary, while organoids offer researchers numerous advantages and opportunities, they also have limitations and cannot yet fully replace other experimental systems.
Whether you are involved in stem cell research, growing organoids, developing a stem cell-based therapy or testing new drugs using stem cells, we offer a complete portfolio of stem cell research products and tools from leading manufacturers that simplify your workflow and enable the continuity, efficiency, and precision that drive discovery. Discover now more about Stem Cell.
References
1. Clevers H. Modeling development and disease with organoids. Cell. (2016) 165:1586–97.
2. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. (2007) 131:861–72.
3. Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, et al. Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes. JCI Insight. (2018) 3:e99941
4. Yui, S. et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat. Med. (2012) 18:618–623.
5. Huch, M. et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. (2013) 494:247–250.
6. Toyohara T, Mae S, Sueta S, Inoue T, Yamagishi Y, Kawamoto T, et al. Cell therapy using human induced pluripotent stem cell-derived renal progenitors ameliorates acute kidney injury in mice. Stem Cells Transl Med. (2015) 4:980–92.
7. Georgakopoulos, N. et al. Long-term expansion, genomic stability and in vivo safety of adult human pancreas organoids. BMC Dev. Biol. (2020) 20: 4.
8. Yoshihara E, O'Connor C, Gasser E, Wei Z, Oh TG, Tseng TW, et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature. (2020) 586:606–11.
9. Hayashi R, Ishikawa Y, Sasamoto Y, Katori R, Nomura N, Ichikawa T, et al. Co-ordinated ocular development from human iPS cells and recovery of corneal function. Nature. (2016) 531:376–80.