Learn More
R&D Systems™ Human IL-35 Fc Chimera, Carrier-Free Recombinant Protein
Extensive quality control produces industry leading bioactivity and lot-to-lot consistency that instills confidence in results and ensures reproducibility.
Brand: R&D Systems™ 8608-IL-050

Description
The Recombinant Human IL-35 Fc Chimera Protein is derived from HEK293. The Recombinant Human IL-35 Fc Chimera Protein has been validated for the following applications: Bioactivity.Specifications
| 10148 | |
| 50μg | |
| Use a manual defrost freezer and avoid repeated freeze-thaw cycles. 12 months from date of receipt, -20 to -70° C as supplied. 1 month, 2 to 8° C under sterile conditions after reconstitution. 3 months, -20 to -70° C under sterile conditions after reconstitution. | |
| IL-35 | |
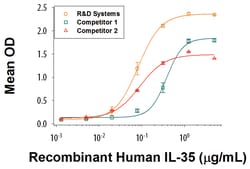
| Measured by its binding ability in a functional ELISA. When Recombinant Human IL-12 R beta 2 Fc Chimera is immobilized at 5μg/mL (100μL/well), the concentration of Recombinant Human IL-35 Fc Chimera that produces 50% optimal binding response is approximately 20-120ng/mL. | |
| Yes |
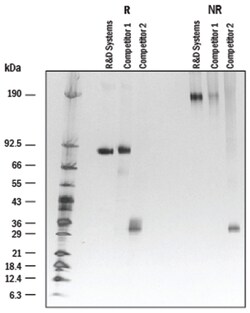
| Observed M.W.: 81-97kDa, reducing conditions, Theoretical M.W.: 73kDa | |
| Human embryonic kidney cell,HEK293-derived human IL-35 protein Human IL-27 beta/EBI3 (Arg21-Lys229) Accession &Num; Q14213 (GGGS)4 Human IL-12 alpha/p35 Arg23-Ser219 Accession &Num; P29459 IEGRMD Human IgG1 Pro100-Lys330) | |
| <0.10 EU per 1μg of the protein by the LAL method. | |
| EBI3 | |
| Unconjugated |
The Fisher Scientific Encompass Program offers items which are not part of our distribution portfolio. These products typically do not have pictures or detailed descriptions. However, we are committed to improving your shopping experience. Please use the form below to provide feedback related to the content on this product.